Every year, hundreds of thousands of patients worldwide face the grim reality of organ failure whether due to heart conditions , kidney failure, chronic liver conditions, or lung disorders. For many, transplantation is the only life-saving option. Yet, the shortage of donor organs remains a persistent, global challenge. According to the World Health Organization, less than 10% of the worldwide need for organ transplantation is currently met.
Traditional solutions expanding donor registries, optimizing organ preservation techniques, and improving transplantation logistics have made an impact, but not enough to solve the crisis. This gap between demand and availability has driven researchers to explore radical new approaches.
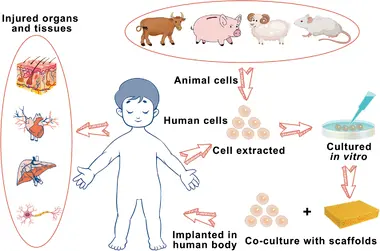
Among the most promising: xenotransplantation, the use of animal organs for human transplantation. But instead of direct animal-to-human transplants, scientists are now developing hybrid technologies that combine xenotransplantation with regenerative medicine. The result? Humanized organs grown within animal-derived scaffolds, potentially offering an abundant, compatible, and personalized source of transplants.
What Are Xenotransplantation + Regenerative Medicine Hybrids?
This approach merges two rapidly advancing fields:
- Xenotransplantation The transplantation of living cells, tissues, or organs from one species to another, most often from pigs to humans due to physiological similarities.
- Regenerative Medicine The use of stem cells, tissue engineering, and biofabrication techniques to repair or replace damaged tissues and organs.
In a hybrid model, the structural framework (scaffold) of an animal organ is used, but it is stripped of all original animal cells. This scaffold composed mainly of extracellular matrix (ECM) proteins like collagen and elastin serves as a biological blueprint. Human stem cells are then introduced, repopulating the scaffold and turning it into a living, humanized organ.
The Science: From Animal Organ to Humanized Transplant
The process can be broken down into a series of precise, technology-driven stages:
1. Decellularization
- The donor animal organ (often pig heart, kidney, or lung) is placed in a specialized perfusion system.
- Detergents and enzymatic solutions remove all animal cells while preserving the 3D architecture of the ECM.
- The result is a ghostly, white, cell-free scaffold with intact vascular channels, valves, and structural components.
2. Sterilization and Preservation
- The scaffold is washed to remove any residual DNA or immunogenic proteins.
- Cryopreservation or chemical stabilization may be used to store scaffolds for future use.
3. Seeding with Human Stem Cells
- Human induced pluripotent stem cells (iPSCs) or mesenchymal stem cells (MSCs) are chosen, often derived from the patient’s own skin or blood cells.
- These cells are coaxed into differentiating into specific organ cell types — cardiomyocytes for hearts, podocytes for kidneys, alveolar cells for lungs.
4. Cultivation in a Bioreactor
- The seeded scaffold is placed in a bioreactor a controlled chamber that mimics the body’s environment.
- Nutrients, oxygen, and growth factors are perfused through the organ’s vascular network to encourage tissue growth and maturation.
5. Pre-Transplant Testing
- Functionality is evaluated: Does the heart contract? Does the kidney filter fluids? Does the lung exchange oxygen?
- Immune compatibility is tested in vitro before clinical transplantation.
Recent Milestones and Research Highlights
- Porcine heart scaffolds with human cardiomyocytes have demonstrated synchronized contractions in laboratory settings, hinting at future functional transplant viability.
- Decellularized pig kidneys repopulated with human renal progenitor cells have achieved partial filtration function in animal models.
- Lung scaffolds seeded with human alveolar epithelial cells have shown improved oxygenation capacity in preclinical trials.
- Researchers at Harvard’s Wyss Institute are integrating CRISPR gene editing before decellularization to further reduce immunogenicity.
Advantages Over Traditional Xenotransplantation
- Lower Immune Rejection – Since the organ is repopulated with the recipient’s own cells, immune suppression requirements are greatly reduced.
- Abundant Supply Animals like pigs could provide a scalable source of organ scaffolds.
- Customizable Organs Patient-specific stem cells allow for tailor-made organs.
- Integration with Other Technologies Gene editing, nanomedicine, and AI-driven bioreactor optimization can enhance outcomes.
The Future Outlook
Within the next two decades, researchers envision:
- “Organ Farms” for Scaffolds Genetically engineered pigs bred to produce immunologically “blank” scaffolds.
- On-Demand Organ Printing Combining natural scaffolds with 3D bioprinting to repair damaged sections of organs rather than replacing the whole organ.
- Smart Organs Integration of biosensors into regenerated organs to monitor function in real time.
- Zoonotic Risk-Free Sources Use of synthetic or plant-based scaffolds shaped to mimic animal organs, avoiding animal pathogens entirely.
Conclusion: A Paradigm Shift in Transplant Medicine
By merging the structural advantages of animal organs with the biological compatibility of human stem cells, xenotransplantation-regenerative medicine hybrids could rewrite the rules of transplantation. If current challenges can be solved, we may one day see a future where organ shortages are eliminated, rejection is rare, and every patient can receive an organ designed specifically for them.
The road ahead is complex scientifically, ethically, and socially but the potential reward is nothing less than a revolution in human healthcare.